- 面议
起订量:
FLUORESCENT CELL MEMBRAN Flipper-TR细胞膜膜张力探针
- 型号
- FLUORESCENT CELL MEMBRAN
- 参数
- 应用领域:医疗卫生
该企业相似产品
世联博研(北京)科技有限公司(Bio Excellence International Tech Co.,Ltd)简称为世联博研。世联博研是一家集进口科研仪器代理销售以及实验技术服务于一体的技术公司。世联博研专注生物力学和3D生物打印前沿科研设备代理销售及科研实验项目合作服务,内容涵盖了血管力学生物学、生物力学建模仿真与应用、细胞分子生物力学、组织修复生物力学、骨与关节生物力学、口腔力学生物学、眼耳鼻咽喉生物力学、康复工程生物力学、生物材料力学与仿生学、人体运动生物力学等生物力学研究以及生物材料打印、打印样品生物力学性能测试分析的前沿领域科研利器和科研服务。
世联博研的客户范围:
科研院所单位、生物医学科研高校、医院基础科研单位等。
世联博研公司代理的品牌具有:
1)近10年长期稳定的货源
2)以生物力学、细胞力学、细胞生物分子学、生物医学组织工程、生物材料学为主,兼顾其他相关产品线
3)提供专业产品培训和销售培训
4)良好的技术支持
5)已成交老客户考证
6)每年新增的货源。
详细信息
Flipper-TR细胞膜膜张力探针
Flipper-TR ® 是一个活细胞荧光膜张力探针,实时地测量活细胞膜张力
Flipper-TR® fluorescent cell membrane tension probe
Flipper-TR®膜张力探针介绍:
荧光膜张力探针Flipper-TR®(Spirochrome, Ltd.)的面世解决了这些挑战,它可以搭配FLIM(荧光寿命成像显微镜)可以实现对活细胞膜的可视化染色。Flipper-TR®是一种活细胞荧光膜张力探针,是di一个为机械生物学领域开发的荧光膜张力探针。Flipper-TR®的荧光寿命与膜张力密切相关,利用FLIM可将不同寿命的荧光转换成不同的颜色显现出来,因此可以通过荧光和颜色及年度即可直观的测量膜内张力的时空分布。Flipper-TR®开辟了一个quan新的领域,它提供了一种灵敏、可靠和无创的方法,通过这种方法可以快速、实时地测量活细胞的膜张力(图1)。
图1. Flipper-TR感知高渗休克处理后的细胞膜张力变化
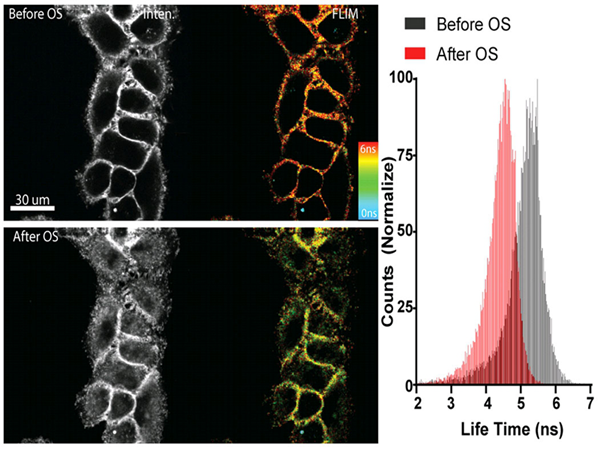
左侧:与 Flipper -TR染色的细胞的图像® 高渗休克之前(上图)和后(下图)。
灰色程度代表荧光强度,不同颜色代表不同荧光寿命(蓝-红:0-6纳秒)
右侧:直方图显示高渗休克后的寿命变化,图片由Colom等人提供。
荧光Flipper -TR ®探针的工作原理是特异性地靶向细胞质膜,通过报告荧光寿命变化来反映膜张力变化情况。它是Flipper探针家族中的成员,它通过机械载体上的两个扭曲的二硫噻吩的扭转角和极化来感知脂质双层膜结构的变化。Flipper -TR ®自发插入到细胞的质膜中,只有插入到脂质膜中才会发出荧光,通过FLIM(荧光寿命成像显微镜)检测荧光的强度及颜色即可判断膜张力的大小,Flipper -TR ®探针具有广泛的吸收和发射光谱; 通常在488nm处进行激发,而发射光谱则在575和625nm之间(图2)。
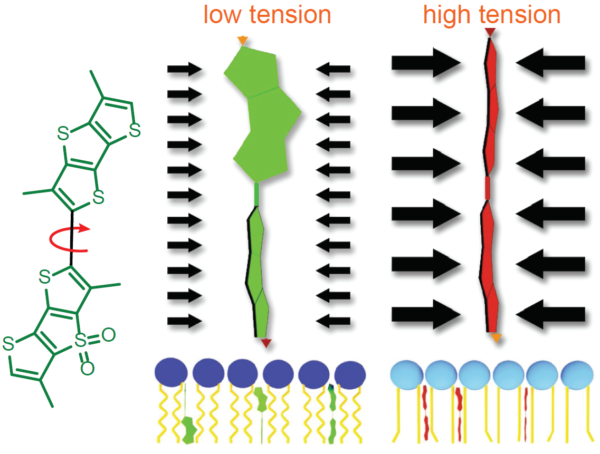
图2. Flipper-TR的工作原理及吸收/发射光谱
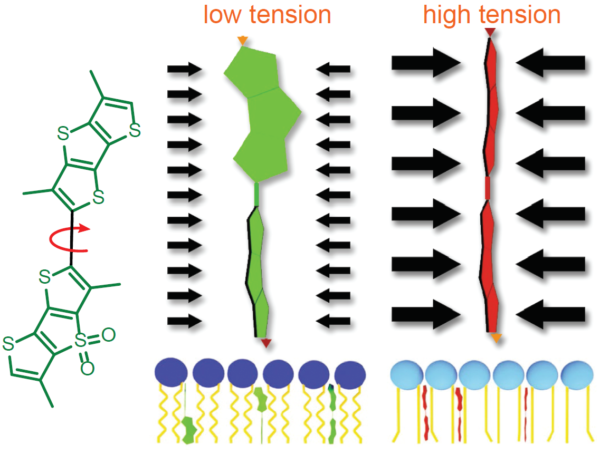
A.Flipper-TR机制及其与不同张力膜相互作用的示意图
左侧:Flipper-TR的基本分子结构
右侧:Flipper-TR在低张力扭曲(绿色)和高张力平行排列(红色)
B.对乙酸乙酯中的Flipper-TR溶液进行扫描和发射扫描,并将结果绘制在同一张图上。
点状橙色线激发光,实心橙色线为发射光。
染色和FLIM成像的详细步骤可以在参考文献中找到(点击了解)。
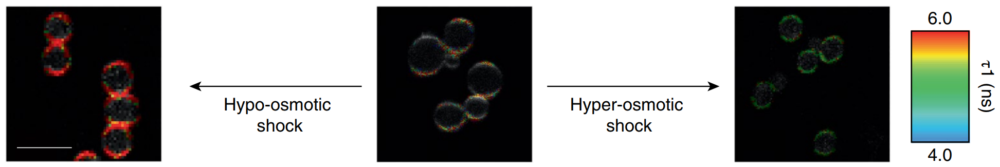
图3 . Flipper-TR在酵母细胞中的染色
Flipper-TR ® 适用于范围广泛的种属:包括细菌,哺乳动物, 植物和酵母。

对Flipper-TR染色的酵母细胞进行低渗透性choc(左)或高渗性choc(右)处理。
Flipper-TR的荧光寿命不同处理条件下高低变化,不同颜色表示不同的荧光寿命。
图片由UNIGE的Roux集团的M. Riggi提供。
Flipper -TR探针的常见问题解答®
- 什么是FLIM显微镜以及它如何用于Flipper-TR?
FLIM显微镜全称荧光寿命成像显微镜(Fluorescent Lifetime Imaging Microscopy)。膜张力研究的重要性在于,在Flipper-TR之前,膜张力测量需要耗费巨大的人力和昂贵的设备,但现在Flipper-TR却可以简单的适配当前显微镜来实现高灵敏度的张力测量,其设备可从许多供应商处获得,它原理是基于记录荧光团激发后发射的时间来反映膜张力的变化,其通常非常快,大约1-10纳秒(ns),在FLIM成像过程中,可将荧光寿命的差异通过时间来展示,一般来说,较短的寿命为绿色,中等寿命为黄色,较长的寿命为橙色和红色。FLIM还可以与其他高分辨率显微技术(如全内反射荧光(TIRF)或受激发射耗尽(STED)显微镜)相结合,用于高空间分辨率检测。
FLIM显微镜需要配备许多科学显微镜供应商提供的时间分辨光检测器,例如PicoQuant的升级套件,参考文献1描述了有关FLIM显微镜实验装置的更多细节。
图4 . FLIM结构和时间分辨彩色编码细胞图像示意图。
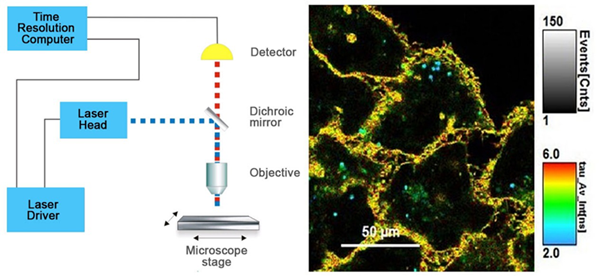
左侧:标准FLIM显微镜结构图
右侧:Flipper -TR®染色的细胞 ,灰度表示荧光强度,不同颜色代表荧光寿命。
图片由Colom等人提供 Flipper-TR是瑞士Spirochrome SA的注册商标。
- Flipper-TR探针如何检测荧光寿命变化?
荧光Flipper-TR®探针通过特异性靶向细胞质膜,并通过其荧光寿命变化来反映膜张力变化。Flipper-TR®自发地插入细胞的质膜中,并且仅在插入脂质膜时才会激发荧光。探针通过机械载体上的两个扭曲的二硫噻吩之间的扭转角和极化改变来感知脂质双层膜结构的变化(参见图5)。当处于紧张状态时(二硫噻吩并排),发射寿命短(2-4ns),而在松弛状态下(二硫噻吩扭曲),发射寿命则更长(4.1-8.0ns)。方差(cv)约为0.3ns(cv = 4-15%),这允许在分辨率细微变化的情况下进行高分辨率分析,在FLIM成像过程中,可将荧光寿命的差异通过时间来展示,一般来说,较短的寿命为绿色,中等寿命为黄色,较长的寿命为橙色和红色(见图5)
图5. Flipper-TR的结构和张力检测机制示意图
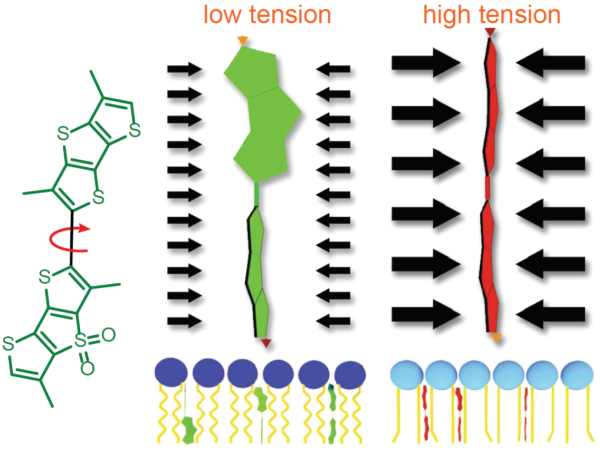
左边:Flipper-TR的基本分子结构
右边:Flipper-TR低张力时扭曲(绿色)和高张力时并列排布(红色)
- 这些探针的滤光片组件是什么?
Flipper-TR探针使用长分离滤光片组进行可视化,因为其激发峰值比发射峰值短100 nm以上。因此,理想的滤光片组是488 +/- 20nm的激发波长和575-675 +/- 40nm的发射波长(图3)。时间分辨测量方法允许非常低的背景,它在水性环境中具有低荧光,详情参见下面的问题4。
- 为什么Flipper-TR探针与其他质膜探针相比具有低背景?
Flipper-TR探针在诸如组织培养基或固定缓冲液等水环境中的背景非常低,因为它是*扭曲的状态,容易形成胶束并自我淬灭(参考文献3)。插入膜后,其扭曲度变小,开始发出高荧光(见图2)。
- Flipper-TR探头在室温下是否稳定?
探针在室温下以粉末形式稳定几天。在无水DMSO中溶解后后(不要使用旧的打开过的DMSO瓶子,可以使用Sigma或Spectrum Chemicals的干燥DMSO瓶子。在-20°C下冷冻和解冻是稳定的,但不建议将其分成小份进行储存,因为它在这些条件下会降解。
- Flipper-TR对细胞有毒吗?
在数据表中给定的条件下操作,探针对细胞无毒的。根据细胞类型和培养条件,细胞一般可存活2-4天, 且荧光亮度不会有太大变化。
- Flipper-TR探针染色哪些生物和组织?
目前所有已知的生物都可用Flipper-TR染色,包括组织培养细胞,活的/固定的组织切片,哺乳动物细胞,昆虫细胞,植物细胞,酵母和细菌。
- Flipper-TR探针是否适用于3D细胞培养?
探针能够在3D培养条件下进行细胞染色。
- 膜中的量子产率和消光系数是多少?
乙酸乙酯中的量子产率= 0.30。
Flipper-TR® fluorescent cell membrane tension probe
 |  |
 |
Flipper-TR®: A Revolutionary New Fluorescent Probe for Measuring Membrane Tension in Cells and Tissues
Flipper-TR® is a live cell fluorescent membrane tension probe which breaks down the technical barriers hitherto circumvented by technical feats known only to biophysicists with custom equipment. The Flipper-TR membrane tension probe simplifies the methodology by using standard fluorescence lifetime measurements (see Flipper-TR FAQ for practical details). Here we describe the background of Flipper-TR in more detail.
Lipid membranes are dynamic, fluid structures (~4 nm thick) which is a biological necessity as they must change shape and tension for a cell to engage in basic cellular and subcellular physiological functions such as migration, cell spreading, phagocytosis, cell division, endocytosis, mechano-transduction, and metabolism, to name but a few. Consequently, membrane tension is under constant regulation due to its required dynamicity, and in turn, membrane tension regulates cell growth, development, motility, endocytosis, and metabolism. As the membrane is remodeled during these cellular processes, bending, tearing, and stretching of the membrane is common. These changes in membrane shape and tension occur over time and in different locations around and inside the cell and are important parameters to measure in order to understand how membrane tension is regulated and how it regulates these various basic, essential cellular processes. Understanding how membrane tension regulates cellular physiology is relevant in the study of healthy and diseased cells.
Membrane tension measurements usually relied on low resolution and slow physical methods to determine forces and tension within the plasma membrane. For example, a standard technique for measuring membrane tension involves pulling on membrane tubes from the plasma membrane with a bead trapped in an optical tweezer – a technique fraught with several methodological and technical limitations. For these reasons, novel, sensitive, reliable, and non-invasive research tools capable of rapidly measuring in vivo changes in membrane tension in real-time are in great demand. The fluorescent membrane tension probe Flipper-TR® (Spirochrome, Ltd.) answers these challenges as it has achieved unparalleled membrane tension sensitivity and temporal resolution through the use of FLIM (fluorescence lifetime imaging microscopy) to visualize Flipper-TR® staining of membranes in living cells. Flipper-TR® is a live cell fluorescent membrane tension probe and the first fluorescent membrane tension reporter developed for the field of mechanobiology. The fluorescence lifetime of Flipper-TR® is strongly dependent on the membrane tension. Using FLIM, the precise measurement of the spatio-temporal distribution of tension in membranes is now possible. Flipper-TR® opens up a whole new field by providing a sensitive, reliable, and non-invasive means by which rapid, real-time membrane tension in live cells is measured (Figure 1).
Figure 1 - Flipper-TR sensing membrane tension in cell undergoing hyperosmotic shock.
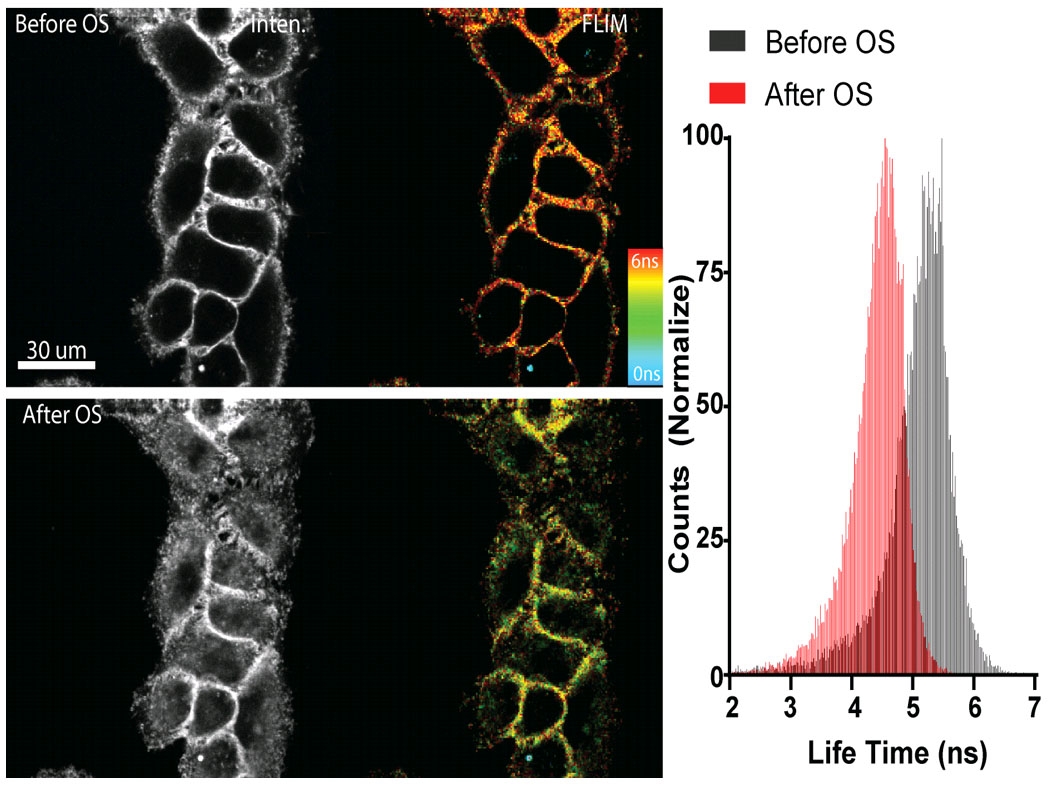
Legend Figure 1: Flipper-TR® staining of cells. Left side: Image of cells stained with Flipper-TR® before (top) and after (bottom) hyperosmotic shock. Greyscale represents fluorescence intensity, and color codes represent fluorescence lifetime. On the right the histogram shows the lifetime shift after osmotic shock. Images courtesy of Colom et al. 2018 (Ref. 1). Flipper-TR is a registered trademark of UNIGEM, Switzerland.
The fluorescent Flipper-TR® probe works by specifically targeting the plasma membrane of cells and reports membrane tension changes through its fluorescence lifetime changes. It is the most advanced member of the Flipper probes family2,3, which sense changes in the organization of lipid bilayer membranes through changes of the twist angle and polarization between the two twisted dithienothiophenes of the mechanophore. Flipper-TR®spontaneously inserts into the plasma membrane of cells and is only fluorescent when inserted into a lipid membrane. It has a broad absorption and emission spectrum; excitation can be commonly performed with a 488 nm laser, while emission is collected between 575 and 625 nm (Figure 2).
Figure 2 - Absorbance and emission spectra of Flipper-TR
A
| 
| B
| 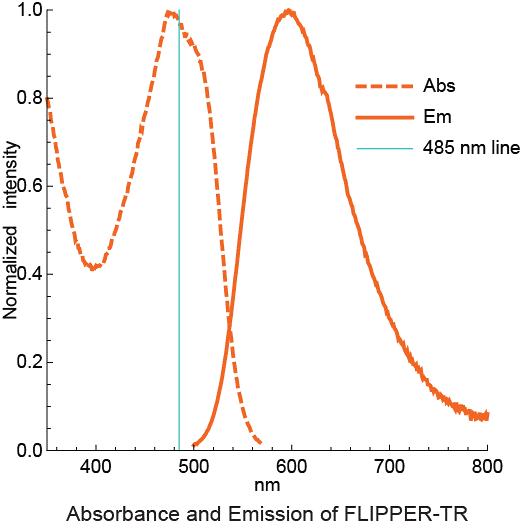 |
Legend Figure 2 - A. Schematic diagram of the mechanism of Flipper-TR and its interaction with membranes with different tension. On the left, basic molecular structure of Flipper-TR. On the right, low tension green twisted Flipper-TR and on the right high tension planar structure. B. Flipper-TR solution in ethyl acetate was subject to absornace and emission scans and the results plotted on the same graph. Absorbance is dotted orange line, and emission is a solid orange line.
A detailed protocol for staining and FLIM imaging can be found below the References (click here, and Ref.5). Flipper-TR® works on a wide range of organisms including bacteria, mammalians, plants, and yeast1,4 (Figure 3).
Figure 3 - Flipper-TR staining in yeast cells
A
|  |
| B |
Legend for Figure 3: Flipper-TR® staining in yeast cells. Yeast cells stained with Flipper-TR and treated with either hypo-osmotic choc (left) or hyper-osmotic choc (right). The fluorescence lifetime of Flipper-TR shifts to high or low values depending on the applied conditions. The color codes for fluorescence lifetime. Images courtesy of M. Riggi from Roux group at UNIGE (Ref. 4). Note the large pseudo-color change which is proprotional to the tension in the membrane.
Data analysis
The photon histograms from ROI or single pixels are fitted with a double-exponential, and two decay times (τ1 and τ2) are extracted. The longest lifetime with the higher fit amplitude τ1 is used to report membrane tension and varies between 2.8 and 7.0 ns. Longer lifetime means more tension in the membrane. τ2 with a smaller value (between 0.5 and 2 ns) and a small fit amplitude is less suited to study membrane tension. The lifetime can be correlated to absolute membrane tension using the calibration procedure given in Ref. 1 (Colom et al. 2018).
Figure 4 - Schematic diagram of short and long lifetime photon release.
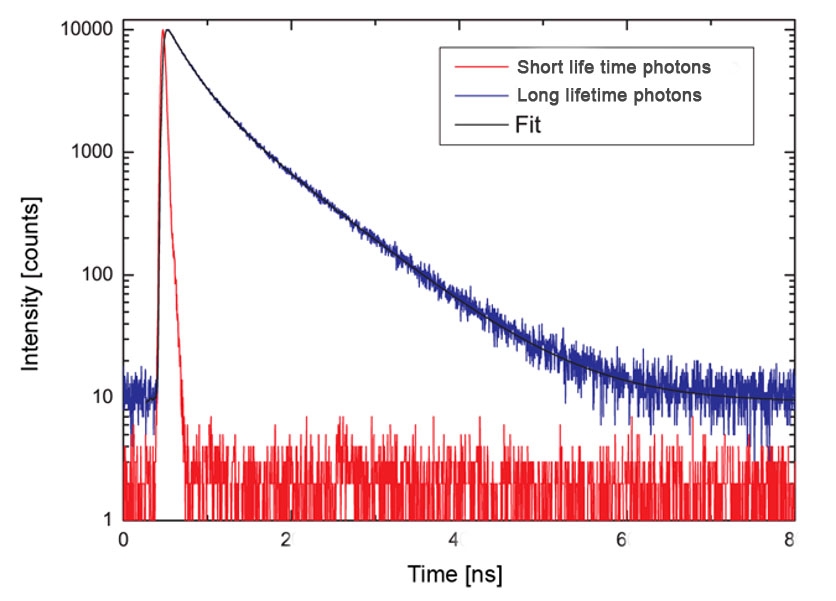
Legend Figure 4 - Short lifetime photons represented by the red dashes. Long lifetime photons represented by the blue dashes. Note - these data are not derived from Flipper-TR but represents the effect of two types of fluorescent lifetime decay.
References
1. Colom A et al. 2018. A fluorescent membrane tension probe. Nat. Chem. 10, 1118–1125.
2. Dal Molin M. et al. 2015. Fluorescent flippers for mechanosensitive membrane probes. J. Am. Chem. Soc. 137, 568-571.
3. Soleimanpour S. et al. 2016. Headgroup engineering in mechanosensitive membrane probes. Chem. Commun. (Camb). 52, 14450-14453.
4. Riggi M et al. 2018. Decrease in plasma membrane tension triggers PtdIns(4,5)P2 phase separation to inactivate TORC2. Nat. Cell Biol. 20, 1043–1051.
5. FLIM microscopy: Lakowicz JR et al. 1994. Emerging biomedical and advanced applications of time-resolved fluorescence spectroscopy. J Fluoresc. 4(1):117-36. doi: 10.1007/BF01876666.
Protocol 1: General Labeling Protocol (consult the datasheet and published papers for detailed protocols)
1. Grow cells on coverslips, glass-bottom dishes, or glass-bottom multi-well plates based on standard laboratory cell culture protocols. When cells have reached the desired density, reconstitute Flipper-TR. Optimal labeling conditions for each cell type should be empirically determined.
2. Reconstitute and prepare a 1 mM master stock solution of Flipper-TR. Store as directed on the datasheet.
3. Prepare a working solution from the 1 mM master stock for staining of the cultured cells. Start with 1 μM stain in cell culture medium. Replace the culture medium with the staining solution.
4. Return the cells to the incubator at 37°C in a humidified atmosphere containing 5% CO2 for 15 minutes before imaging.
5. Image stained cells with standard FLIM techniques using a 485 or 488 nm pulsed laser for excitation and collecting photons through a 600/50 nm bandpass filter. Optimization of the labeling procedure and image acquisition settings is recommended so that photodamage is minimized. NOTE: Membrane tension measurements can only be performed by FLIM (fluorescence intensity or wavelength are not reliable).
Data Analysis
For extraction of lifetime information, the photon histograms from ROI or single pixels are fitted with a double-exponential, and two decay times (τ1 and τ2) are extracted. The longest lifetime with the higher fit amplitude τ1 is used to report membrane tension and varies between 2.8 and 7.0 ns. Longer lifetime means more tension in the membrane. τ2 with a smaller value (between 0.5 and 2 ns) and a small fit amplitude is less suited to study membrane tension. The lifetime can be correlated to absolute membrane tension using the calibration procedure given in reference # 1 (Colom et al. 2018).









